CASE REPORT |
https://doi.org/10.5005/jp-journals-10002-1426 |
A Collision Tumor of Dermatofibrosarcoma Protuberance with Anaplastic Thyroid Carcinoma
1Department of Surgery, Universiti Sains Islam Malaysia, Nilai, Negeri Sembilan, Malaysia
2,3Department of Surgery, Universiti Kebangsaan Malaysia, Cheras, Kuala Lumpur, Malaysia
4Department of Pathology, Universiti Kebangsaan Malaysia, Cheras, Kuala Lumpur, Malaysia
Corresponding Author: Rohaizak Muhammad, Department of Surgery, Universiti Kebangsaan Malaysia, Cheras, Kuala Lumpur, Malaysia, Phone: 603-91455555, e-mail: rohaizak@hotmail.com
ABSTRACT
Anaplastic thyroid cancer (ATC) is the most aggressive with the worst prognosis of thyroid malignancies. The malignant transformation from preexisting differentiated thyroid cancer (DTC) remains ambiguous. Another rare cutaneous tumor known as dermatofibrosarcoma protuberans (DFSP) has a low metastatic rate yet a high risk of recurrence. DFSP requires diagnosis by pathological examination, and the main treatment is surgical resection with a good negative margin. We describe a young patient with malignant transformation of ATC from differentiated papillary thyroid cancer with the collision of DFSP variants. A 42-year-old lady presented with left neck swelling, which developed 6 months after total thyroidectomy and bilateral lymph node dissection for papillary thyroid carcinoma (PTC). Despite radioiodine ablation after surgery, the swelling progressively increased in size. It involved the overlying surrounding skin and previous thyroidectomy scar. Serum thyroglobulin was high, with low antithyroglobulin suggestive of local recurrence. A tissue biopsy of the lesion confirmed the anaplastic transformation of PTC. She has unilateral left vocal cord palsy due to disease progression. Further fluorodeoxyglucose-positron emission tomography (FDG-PET) scan revealed a positive uptake only in the left thyroid bed. She underwent debulking surgery, tracheostomy, and percutaneous endoscopic gastrostomy, given her young age and good performance status. The final histopathology results showed a collision tumor of DFSP with anaplastic thyroid carcinoma. The disease progressed, and she succumbed to the disease 6 months after surgery. DFSP variants and ATC are two rare kinds of tumors. There are a few reports in the literature on metastatic DFSP variants to the thyroid gland. The diagnosis of ATC or even DFSP is important as the management will be different in the intent of therapy. We report a case of these collision tumors for their rarity and difficulty in their management.
How to cite this article: Jamaluddin NN, Muhammad R, Abdullah SSN, et al. A Collision Tumor of Dermatofibrosarcoma Protuberance with Anaplastic Thyroid Carcinoma. World J Endoc Surg 2022;14(1):31-33.
Source of support: Nil
Conflict of interest: None
Keywords: Anaplastic thyroid cancer, Dermatofibrosarcoma, Protuberans, Recurrence, Sarcomatoid
INTRODUCTION
Anaplastic thyroid cancer (ATC) is the deadliest thyroid solid tumor in humans because of its aggressiveness and worst prognosis. It is rare, constituting less than 2% of thyroid carcinomas. The best evidence has shown that most ATC arise from the carcinogenic malignant change that originates from preexisting DTC. Yet, the carcinogenic mechanism of anaplastic transformation stays undefined.
Dermatofibrosarcoma protuberans (DFSP) variants is a low-grade soft tissue tumor that contributes to 1% of all soft tissue sarcomas.1,2 It occurs in the dermis and subcutaneous tissues. It has a unique histologic feature, but other diseases can also be imitated by it. Histologically, several DFSP variants were defined, and it is crucial to distinguish them to avoid misdiagnosis for the subsequent appropriate treatment and prediction of clinical outcomes. We describe a case of ATC with the occurrence of a DFSP variant in a young patient. The final histology showed the anaplastic transformation from a differentiated thyroid carcinoma with a DFSP variant.
CASE DESCRIPTION
A 40-year-old female patient with an unknown medical illness was referred for recurrent left neck swelling. She was diagnosed with PTC and went through total thyroidectomy with bilateral central lymph node dissection. This was followed by radioiodine ablation postoperatively. She was well throughout the surveillance until 6 months after radioiodine ablation, she developed a left neck swelling, which was associated with hoarseness of voice. The left neck swelling was hard and fixed to the overlying skin and muscle of the previous thyroidectomy scar. Serum thyroglobulin was elevated with low antithyroglobulin consistent with local recurrence. A neck ultrasound revealed a left thyroid bed recurrence with multiple cervical lymph nodes.
Clinical assessment of the neck shows left anterior neck swelling with multiple cervical lymph node enlargements. It was hard and fixed to the underlying muscle (Fig. 1). Preoperative indirect laryngoscope shows left vocal palsy with fullness in the left pyriform fossa. Her thyroid status also was normal.

Fig. 1: A recurrence of neck swelling with skin nodule involvement after completion of thyroidectomy and lymph node dissection
Ultrasound-guided FNAC showed the presence of bizarre cells suggestive of anaplastic carcinoma. Contrast-enhanced computed tomography (CECT) thorax, abdomen, and pelvis showed bilateral multiple lung metastases with thoracic metastasis (Fig. 2). The diagnosis of malignant transformation of anaplastic thyroid carcinoma from a previously distinguished thyroid carcinoma has been made. The scan of the FDG-PET exhibited positive uptake in the left thyroid bed, which is a locoregional disease without distant metastasis.

Fig. 2: CECT neck and thorax showing local recurrence in the left thyroid bed and locally invasive (arrow)
Since the patient is a young patient with good performance status and an absence of distant metastasis, she underwent a debulking surgery with tracheostomy and percutaneous gastrostomy. Intraoperatively, the left thyroid bed lesion was densely adhered to the underlying strap muscles and also overlying skin. The left carotid artery was completely encased with trachea infiltration. Subsequently, she had palliative radiotherapy and succumbed to death 6 months after surgery. Histopathology with immunohistochemistry confirmed the presence of anaplastic thyroid carcinoma with the sarcomatoid variant as well as high-grade DFSP of the skin (Figs 3A to H).
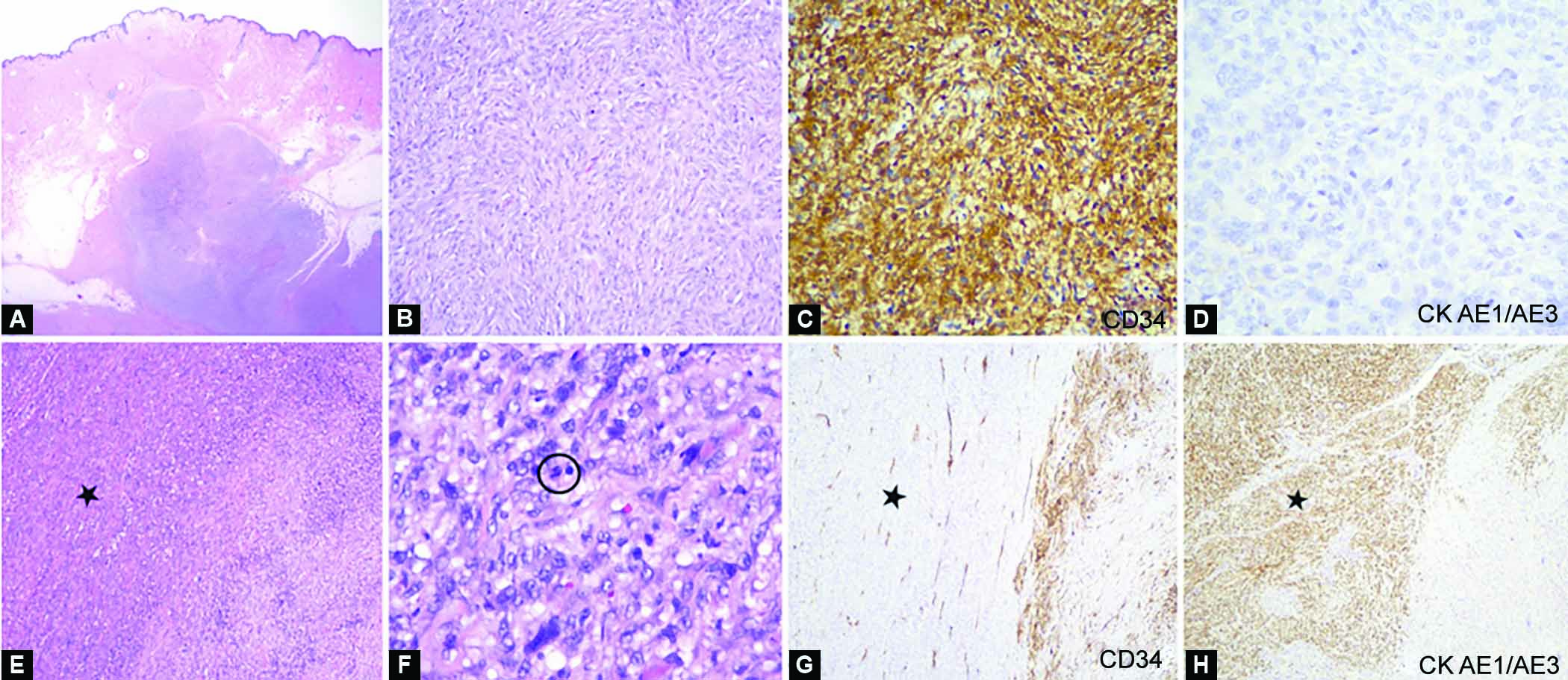
Figs 3A to H: Photomicrograph of collision tumors: Dermatofibrosarcoma protuberans and anaplastic thyroid carcinoma. (A) Low power view of a dermal tumor, formed of malignant spindle cells infiltrating through subcutaneous fat (H&E, x10); (B) The malignant spindle cells are structured in the pattern of tight storiform, displaying pleomorphic vesicular nuclei and moderate cytoplasm (H&E, x200); (C) Immunohistochemically, the cells of the malignant spindle are diffusely positive for CD34 (CD34, x200); (D) are CK AE1/AE3 negative (CK AE1/AE3, x400), consistent with the diagnosis of dermatofibrosarcoma protuberans; (E) In other areas, a second population (black star) of malignant spindle cells are noticeable (H&E, x100); (F) They display moderate to marked pleomorphic, hyperchromatic to vesicular nuclei, some with prominent nucleoli. Mitosis is occasionally seen (circle) (H&E, x400); (G) The second population (black star) of malignant spindle cells are negative for CD34 (CD34, x100); (H) Demonstrates CK AE1/AE3 diffuse positivity (CK AE1/AE3, x100), consistent with the diagnosis of anaplastic thyroid carcinoma, sarcomatoid variant
DISCUSSION
The rarest yet with the worst prognosis is thyroid cancer, known to be ATC. It commonly occurs in people over the age of 60 years. It often presented with the rapid growth of neck mass with local compressive or infiltrative symptoms. Studies have shown that ATC derives from both DTC and poorly DTC (PDTC) since they may sustain the mutations of the tumor they derive from. Histologically, the ATC does not show any morphological features of follicular cells.3
Most ATC occurs in older patients, particularly those with a long-standing thyroid tumor. Hence, it could be the root cause of the occurrence of anaplastic transformation. In the case presented, she was first diagnosed with PTC at the age of 40. Subsequently, 6 months after thyroidectomy and bilateral central lymph node dissection, and completion of single radioiodide ablation, she developed a local recurrence in the left thyroid bed, which also involved the overlying skin nodule. A guided biopsy of the left thyroid bed recurrence showed a malignant transformation of ATC from previously proven PTC.
The multimodal therapy for ATC comprises surgery, radiotherapy, and chemotherapy. Systemic treatment with kinase inhibitors has demonstrated a prolonged survival, especially in metastatic disease. Despite the multimodality approach of ATC, its prognosis is still dire. The average survival rate for ATC with metastatic disease is 3–6 months. In this patient, the progression of malignant transformation of ATC has been on 6 months after initial curative surgery with subsequent radioiodide ablation.
The 8th version of the published American Joint Committee on Cancer/tumor node metastasis (TNM) staging systems have categorized ATC as a T4 tumor, which is defined as tumor extension beyond the thyroid gland regardless of tumor size.4 The stage for ATC is labeled as stage IV due to its systemic disease. When the ATC is only intrathyroidal is then divided into 4A (IVA), while 4B (IVB) in a gross extrathyroidal extension or cervical lymph node metastases, and 4C (IVC) with the presence of distant metastasis, respectively. This patient is already in stage IVC, but in view of her young age and good performance status, debulking surgery with tracheostomy and percutaneous gastrostomy is the best option for her with palliative intent.
The approach to ATC is now shifting toward personalized medicine, tailored to the clinical characteristic and genetic profile of the patient. In general, no method can predict a thyroid neoplasm that will undergo anaplastic transformation. The etiology of anaplastic transformation in thyroid tumorigenesis remains unclear. Some literature has recommended genetic profiling of BRAF mutation analysis since systemic treatment with dabrafenib and trametinib are preferred in BRAF mutation.5 Others have presented that Tp53 gene mutations are usually present in anaplastic tumors but are seldom found in DTCs.6,7 In this case, molecular testing on microsatellite instability containing MutS homologue (MSH), Mut L homologue (MLH), post meiotic segregation homologue (PMS), cellular surface receptor tyrosine kinase CD117, CD = cluster of differentiation (Ckit/CD117) were all negative. There was no further genetic testing done due to financial constraints. Therefore, she was given palliative chemotherapy in a combination of cisplatin-based (Doxorubicin) and Ifosfamide for both ATC and sarcoma despite a tyrosine kinase inhibitor.
Meanwhile, a rare soft tissue tumor known as DFSP is categorized as a sarcoma of intermediate-grade malignancy for its low rate of metastasis yet aggressive local invasion. The cause of DFSP is still unclear. A chromosomal translocation was involved in several studies, leading to the fusion protein of COL1A1-PDGFB to tumor growth through the mass development of the platelet-derived growth factor (PDGF).8 There have been reports on DFSP also occurs in preexisting scars and tattoos. In the case presented, this patient had a previous thyroidectomy scar and subsequently underwent another surgery of a similar collar incision.
Sarcomatoid ATC is known to simulate some soft tissue sarcomas. It is a diagnostic challenge to differentiate sarcomatoid anaplastic carcinoma from a true sarcoma in the absence of distinguished components and inability of immunohistochemistry to display an epithelial differentiation. Two histological features that help to distinguish the sarcomatoid anaplastic carcinoma from the actual sarcoma are: (1) the existence of angulated necrotic foci with neoplastic cell palisading surrounding them as viewed in glioblastoma of the central nervous system and (2) the possibility of the spindle neoplastic cells to penetrate the large arteries and the vein wall.8
Primary thyroid sarcoma is extremely rare, and it has been suggested that sarcomatoid tumors of the thyroid gland should be considered ATC.9 Some primary sarcomas simulate the sarcomatoid ATC that have been reported, such as angiosarcoma, chondrosarcoma, fibrosarcoma, leiomyosarcoma, and osteosarcoma. Metastasis is also possible and necessary to be ruled out clinically.9,10
Besides, the neoplastic and non-neoplastic spindle cells in thyroid lesions can simulate a sarcomatoid pattern. This is challenging for the pathologist; thus, further evaluation of histopathology with immunohistochemistry helps to establish the correct diagnosis.
CONCLUSION
Malignant transformation of anaplastic from well-DTC tumor is extremely rare in a young patient. The concurrence of ATC with DFSP variants has not been reported to date.
REFERENCES
1. DuBay D, Cimmino V, Lowe L, et al. Low recurrence rate after surgery for dermatofibrosarcoma protuberans: a multidisciplinary approach from a single institution. Cancer 2004;100(5):1008–1016. DOI: 10.1002/cncr.20051
2. Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Semin Diagn Pathol 2013;30(1):13–28. DOI: 10.1053/j.semdp.2012.01.002
3. Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: Kinase inhibitors and beyond. Endocr Rev 2019;40(6):1573–1604. DOI: 10.1210/er.2019-00007
4. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (Eighth Edition): what changed and why?. Thyroid 2017;27(6):751–756. DOI: 10.1089/thy.2017.0102
5. De Leo S Trevisan M, Fugazzola L. Recent advances in the management of anaplastic thyroid cancer. Thyroid Res 2020;13(1):17. DOI: 10.1186/s13044-020-00091-w
6. Nakamura T, Yana I, Kobayashi T, et al. p53 gene mutations associated with anaplastic transformation of human thyroid carcinomas. Jpn J Cancer Res 1992;83(12):1293–1298. DOI: 10.1111/j.1349-7006.1992.tb02761.x
7. Fagin JA, Matsuo K, Karmaker A, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 1993;91(1):179–184. DOI: 10.1172/JCI116168
8. Ragazzi M, Ciarrocchi A, Sancisi V, et al. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol 2014;2014:790834. DOI: 10.1155/2014/790834
9. Carcangiu ML, Steeper T, Zampi G, et al. Anaplastic thyroid carcinoma. A study of 70 cases. Am J of Clin Pathol 1985;83(2):135–158. DOI: 10.1093/ajcp/83.2.135
10. Rosai J, Carcangiu ML. Pitfalls in the diagnosis of thyroid neoplasms. Pathol Res Pract 1987;182(2):169–179. DOI: 10.1016/S0344-0338(87)80100-0
________________________
© The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.